VivaChek receives CE mark for VivaChek™ Ino glucose system
Update: 2015-04-10VivaChek today announced that it has received CE Mark (Conformité Européenne) for its VivaChek™ Ino Blood Glucose Monitoring System from TUV Reinland, together with the ISO 13485 certificate for the manufacturing facility. VivaChek™ Ino is an innovative no coding and very user-friendly system meets ALL the requirements of the EN ISO 15197:2013 standard.
According to the International Diabetes Federation, there are 382 million people around the globe living with diabetes, more than 56 million of whom live in Europe, and that number is projected to increase by more than 20 percent by the year 2035 1 .
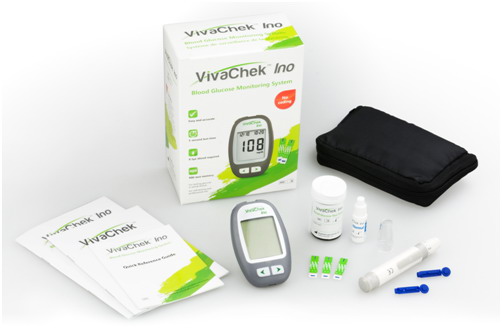
Key features of VivaChek™ Ino System include:
• 0.5µL blood sample, about 5s testing time
• No Coding technology, less test steps, more test confidence
• Strip ejector discards the used strip smoothly to avoid contamination
• 8 electrodes test strip ensures accurate test
• Unique hematocrit (Hct) correction technology allows to test sample Hct range from 20- 70%
• 900 meter memory with data and time, 7,14 and 30 day averaging
• Optional operation mode for selection, L-1 for simple setting, L-2 for advanced setting
Click HERE for detailed information of VivaChek™ Ino
Reference:
1. IDF Diabetes Atlas; sixth edition www.dif.org/diabetesatlas

