VivaDiag™ SARS-CoV-2 IgM/IgG Rapid Tests have been evaluated by FIND and KU Leuven
Update: 2020-07-10Hangzhou & San Diego, July 10, 2020 - in-vitro diagnostic manufacturer VivaChek announced today that the VivaDiag™ SARS-CoV-2 IgM/IgG Rapid Tests have been evaluated by FIND and KU Leuven Belgium recently.
On the 13 th of March 2020, FIND launched an expression of interest (EOI) process for SARS-CoV-2 antigen or antibody tests developers to have their immunoassays evaluated independently by standardized protocols. The EOI was closed on the 20th of March 2020, with over 100 submissions received.
Recently FIND published the result of the first 4 Antibody tests including our VivaDiag™ SARS-CoV-2 IgM/IgG Rapid Test.
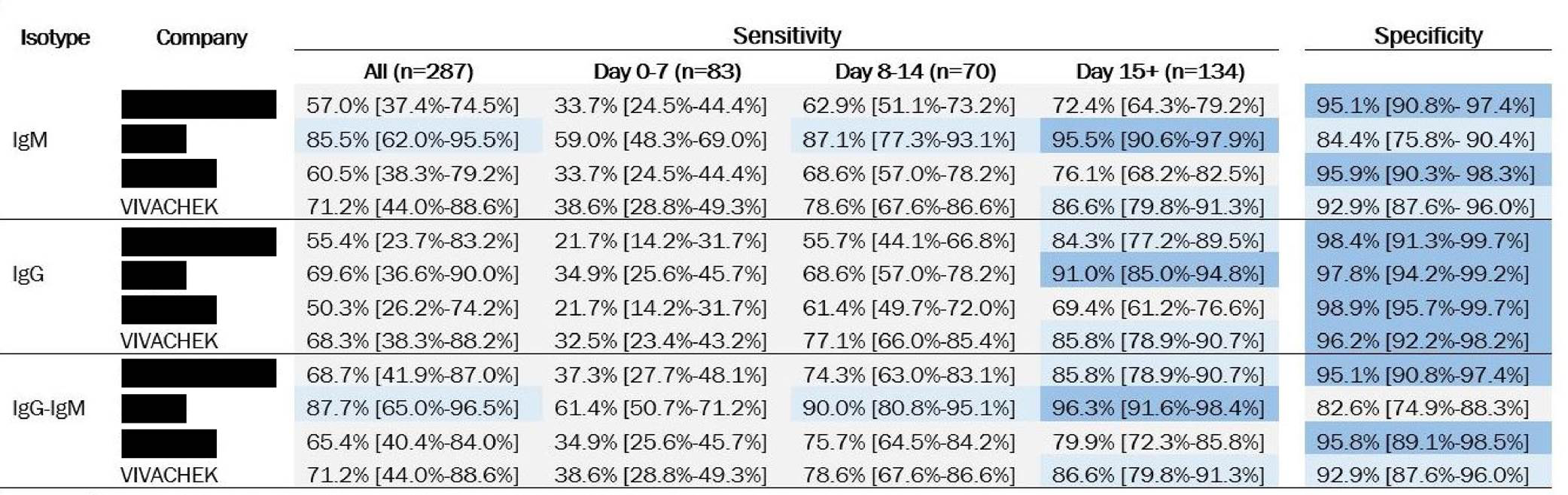
The summary of the evaluation is accessible at FIND's website
https://www.finddx.org/covid-19/sarscov2-eval-immuno/immuno-eval-results/
Note: More product-specific performance point-estimates will be posted as data become available.
One important message to notice is that our product was selected by FIND among more than 100 suppliers for the first batch. Such recognition not only proves the high performance of our product, but also indicates the well-established quality management system (QMS) and production capacity at VivaChek.
Furthermore, VivaDiag™ SARS-CoV-2 IgM/IgG Rapid Tests were evaluated in University Hospitals Leuven and KU Leuven, Belgium. The results [1] further provide well-sounded data indicating the superior performance of our products.
Till June 2020, VivaDiag™ SARS-CoV-2 IgM/IgG Rapid Tests have been exported to more than 50 countries and obtained certificates of ISO 13485:2016, CE and registration at Netherland CIBG, UK MHRA, Australia TGA, Singapore HSA, Saudi MDAM and authorities from Russia, Ukraine, Colombia, Peru and so on.
Please visit our website for further information:
https://www.vivachek.com/en/prods/prod-rapidtest.html
About VivaChek
VivaChek is a professional in-vitro diagnostic manufacturer with head office in the USA and manufacture facility in China. VivaChek manufacturers and delivers millions of tests of blood glucose, blood ketone, immunoassay rapid tests etc every year to more than 120 countries including USA, China, Germany, Italy etc, and most products are CE marked and FDA 510k cleared.
Reference
[1] J. Van Elslande et al., “Diagnostic performance of 7 rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients,” Clin. Microbiol. Infect., May 2020, doi: 10.1016/j.cmi.2020.05.23.
