

SARS-CoV-2 RT-PCR Detection Kit
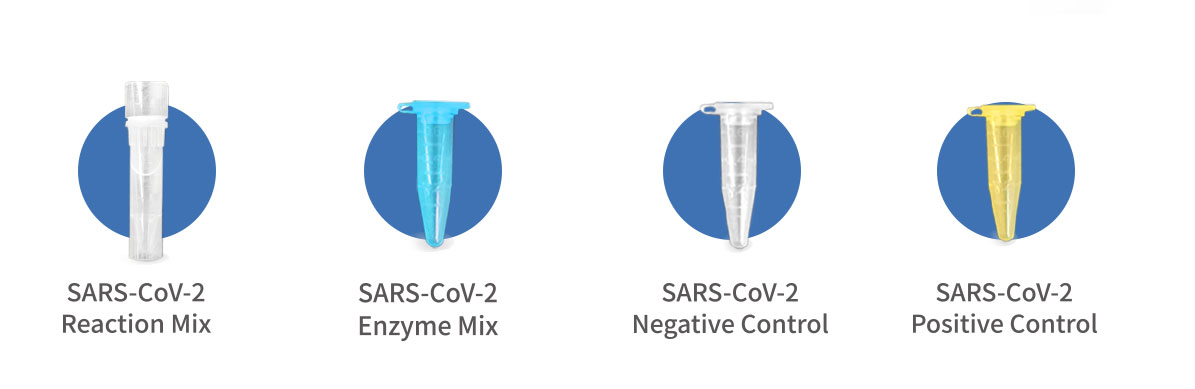
| Ref | Component | Presentation 50rxns |
|---|---|---|
| 1 | SARS-CoV-2 Reaction Mix | 1 tube, 950 μL |
| 2 | SARS-CoV-2 Enzyme Mix | 1 tube, 50 μL |
| 3 | SARS-CoV-2 Negative Control | 1 tube, 50 μL |
| 4 | SARS-CoV-2 Positive Control | 1 tube, 50 μL |

Product Features
-
Double-target detection of genes to improve diagnosis accuracy
The ORF1ab gene of SARS-Cov-2 will be detected by FAM channel, and the N gene ofSARS-CoV-2 will be detected by ROX channel.
-
Negative and positive controls provide calibration for the kit
Internal reference is used in the kit for quality control starting from sample collection.
-
Use anti-contamination system to prevent product contamination
dUTP and UNG enzyme are used in the kit to prevent contamination of the amplifiedproducts.
-
With great clinical performance
Clinical evaluation study was performed to evaluate the performance of the SARS-Cov-2RT-PCR Detection Kit. Compared with the comparator method, the PPA was 99.07%, andthe NPA was 98.97%.
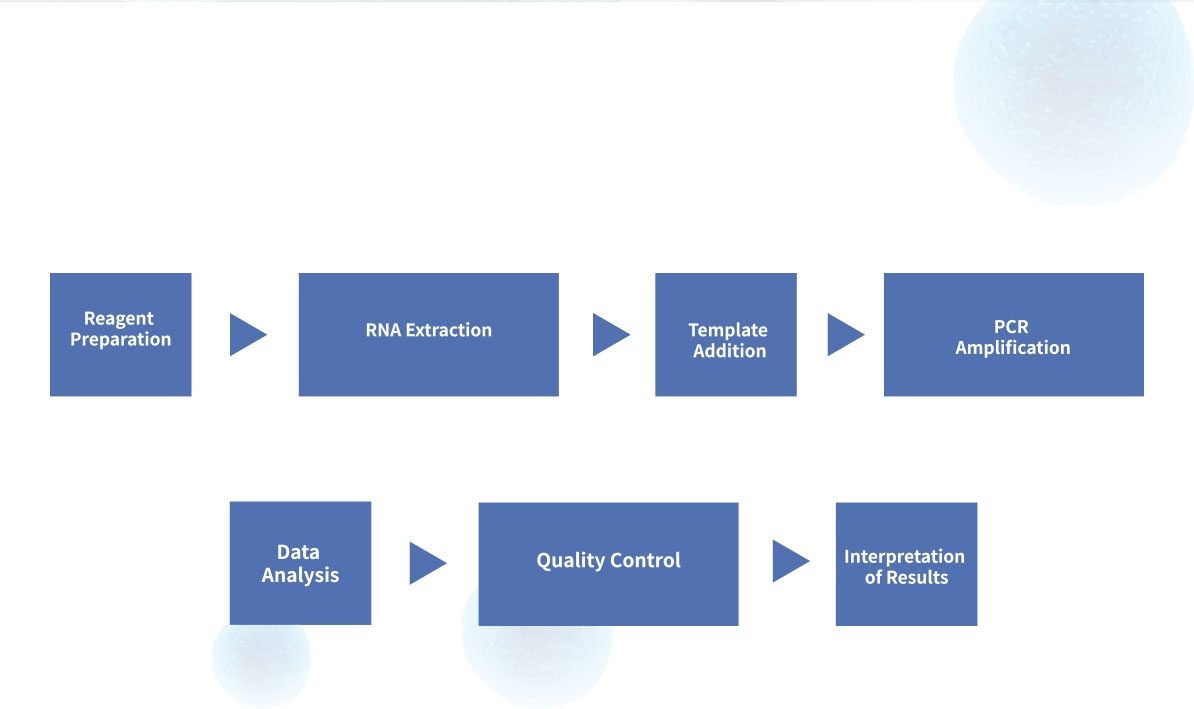
Steps for Simple Test
Specification
| Performance | Sensitivity: 200 copies/mL |
| Test Principle | RT-PCR |
| Sample Type | Throat (oropharyngeal) swabs, nasopharyngeal swabs, anterior nasal swabs, mid-turbinate nasal swabs, nasal washes, nasal aspirates and bronchoalveolar lavage fluid (BALF) |
| Target Genes | ORFlab, N |
| Test Time | 70 min |
| Operation Temperature | Room temperature |
| Storage Temperature | -25℃ to -15℃ |
| Shelf Life | 12 months |
Not Available for Sales in the US
**Warning**
NOTE: Test results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable respiratory specimens during the acute phase of infection. Positive results are indicative of the presence of SARS-CoV-2 RNA. Clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.