

SARS-CoV-2 Ag FIA Test

Advanced Technical Features
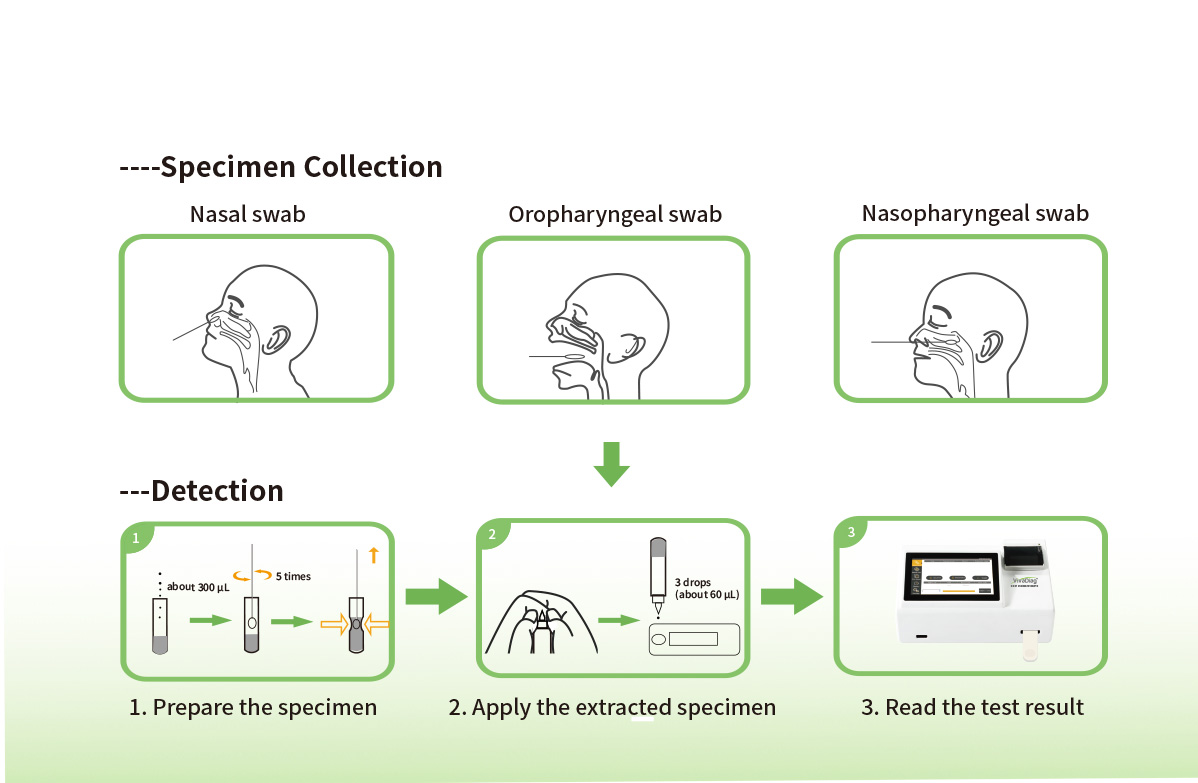
Steps for Simple Test
Specification
----FIA Analyzer
| Model | VIM1000 |
| Size | 280×240×130 mm |
| Screen | 7″ LCD touch screen |
| Connectivity | RS232 & USB & COM |
| Printer | Built-in thermal printer |
| Memory | 50000 test results |
| Model | AFS-1000 |
| Size | 215×303×159 mm |
| Screen | 7″ LCD touch screen |
| Connectivity | USB, ethernet port, serial port, double serial port, WiFi (optional) |
| Printer | Built-in thermal printer |
| Model | VIM2000 |
| Size | 192×205×161 mm |
| Screen | 7″ LCD touch screen |
| Connectivity | Ethernet port RJ-45 & USB |
| Incubator | Built-in |
| Printer | Built-in thermal printer |
----Test Kit
| Test Principle | Fluorescence immunochromatography |
| Sample Type | Nasal swab,oropharyngeal swab or nasopharyngeal swab |
| Sample Volume | 60 μL |
| Test Time | 15 min |
| Operation Temperature | 15-30℃ |
| Storage Temperature | 2-30℃ |
| Shelf Life (Unopened) | 24 months |
Not Available for Sales in the US
**Warning**
NOT FOR AT-HOMETESTING
VivaDiag™ SARS-CoV-2 Ag FIA Test has ONLY been designed to act as a supplementary test for suspected cases of negative coronavirus nucleic acid detection or in conjunction with nucleic acid detection in the diagnosis of suspected cases. Results from nucleocapsid protein antigen testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 (COVID-19) infection or to inform infection status.