
VivaDiag™ SARS-CoV-2/Flu A/Flu B Ag Rapid Test

Advanced Technical Features
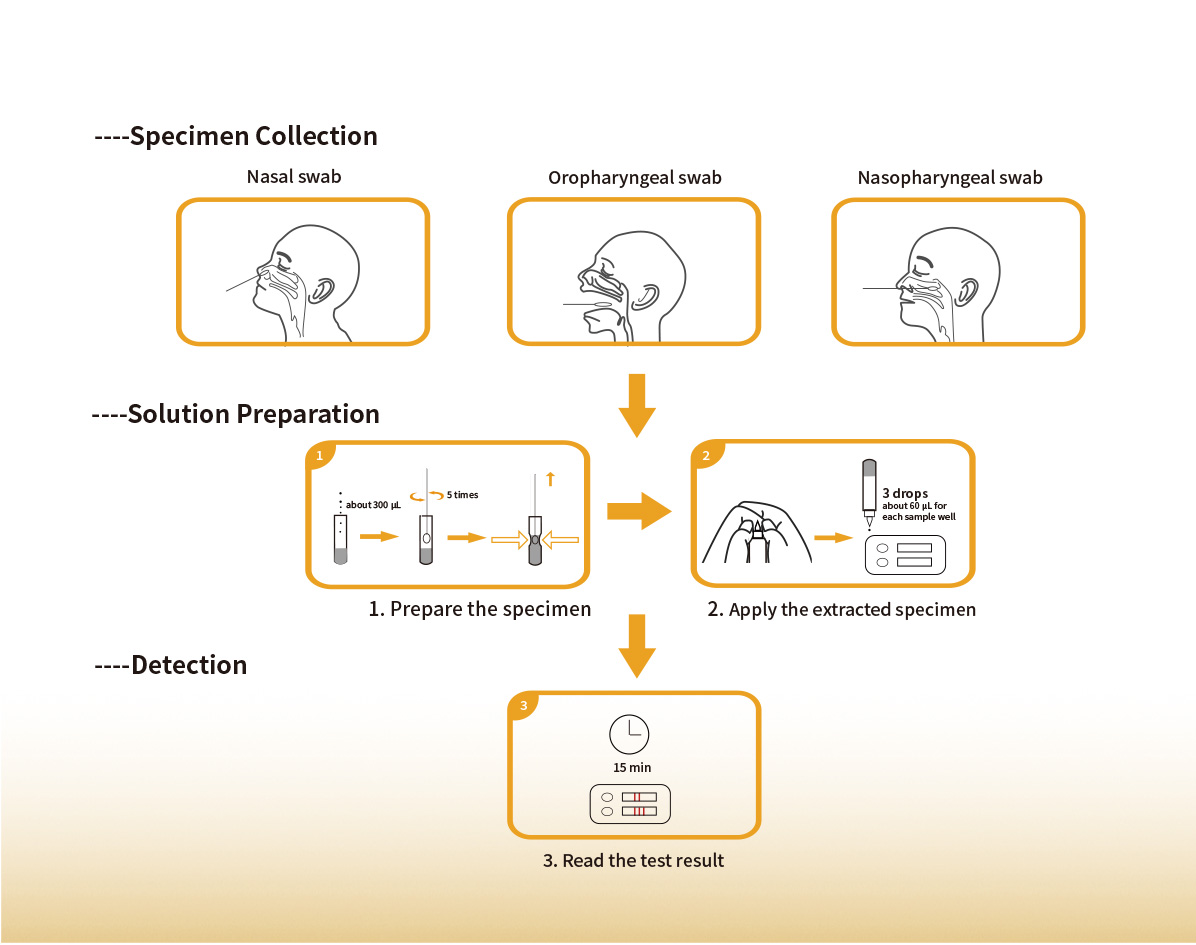
Steps for Simple Test
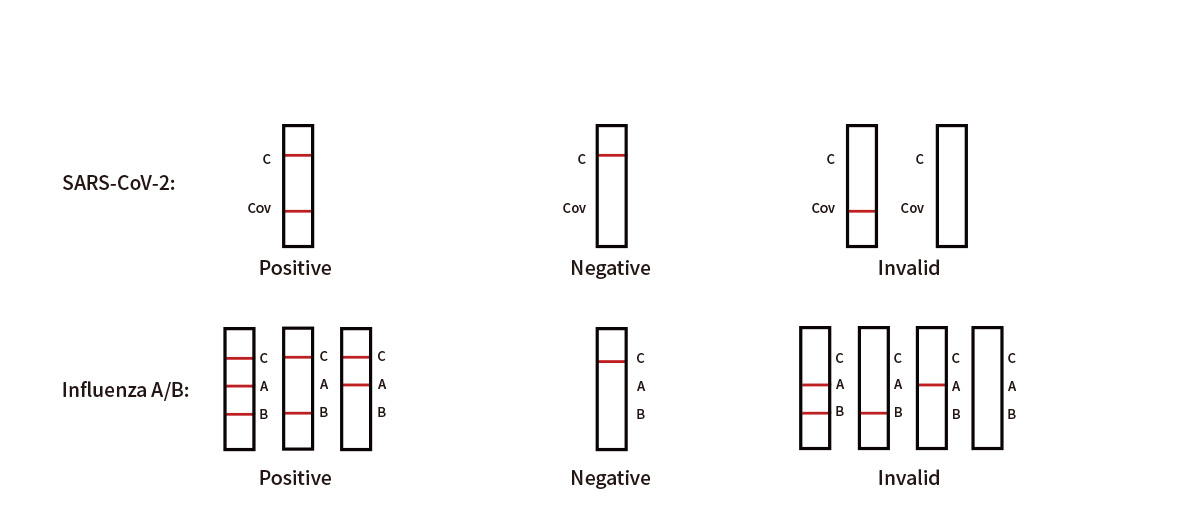
Result Interpretation
Specification
| Test Parameters | SARS-CoV-2 Ag /Flu A Ag /Flu B Ag |
| Test Principle | Colloidal gold |
| Sample Type | Nasal swab,oropharyngeal swab or nasopharyngeal swab |
| Sample Volume | 60μL (each sample well) |
| Test Time | 15 min |
| Operation Temperature | 15-30℃ |
| Storage Temperature | 2-30℃ |
| Shelf Life | 24 months |
Not Available for Sales in the US
**Warning**
NOT FOR AT-HOMETESTING
Note: The VivaDiag™ SARS-CoV-2/Flu A/Flu B Ag Rapid Test has ONLY been designed to function as a supplementary tool in conjunction with nucleic acid test for the diagnosis of suspected cases of SARS-CoV-2, influenza A or influenza B. Thus, the results from rapid test should not be used as the sole basis to diagnose or exclude SARS-CoV-2, influenza A or influenza B infection. Furthermore, the test results should not be used to determine or inform the infection status.