Adenovirus Nucleic Acid Detection Kit
VivaDiag™ Adenovirus Nucleic Acid Detection Kit is designed for in-vitro detection of Respiratory Adenovirus in nasal swab or throat swab specimens. Test results of the diagnostic kit are used for clinical reference, other relevant medical examination results should be considered for comprehensive analysis. Test results should not be used as a sole basis for clinical diagnosis and negative results cannot preclude infections.
controls provide
calibration for the kit
and specificity;
No cross-reactivity
enzyme are used
Operation procedures
 1.
Sample collection
1.
Sample collection
 2.
Nucleic acid extraction
2.
Nucleic acid extraction
 3.
PCR amplification
3.
PCR amplification
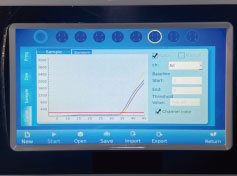 4.
Result analysis
4.
Result analysis
Specification
| Performance | Sensitivity:500 copies/mL; Precision:CV<5% |
| Test Principle | Real-time PCR |
| Sample Type | Nasal swab or throat swab |
| Target Genes | Adenovirus DNA |
| Test Time | 70 min |
| Operation Temperature | Room temperature |
| Storage Temperature | -25℃ to -15℃ |
| Packing Size | 48T/Kit, 96T/Kit |
| Shelf Life | 12 months |
Amplification and detection instruments
Applied Biosystems™ 7500 Real-Time PCR System; Applied Biosystems™ 7500 Fast Real-Time PCR System; Roche LightCycler® 480 System; Bio-Rad CFX96 Real-Time System; Hongshi SLAN®-96P Real-Time PCR System; Real-Time PCR Detection System AGS8830; Rotor-Gene Q 5plex Platform; Tianlong Gentier 96E Real-Time PCR System; Bioer technology FQD-96C